ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Science Videos 686 videos
In this video, we dive beneath the sea to review the kinds of interesting animals that live in the deep blue.
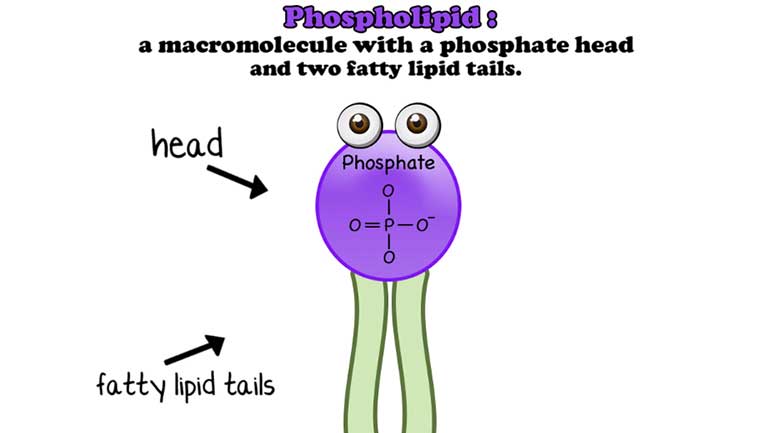
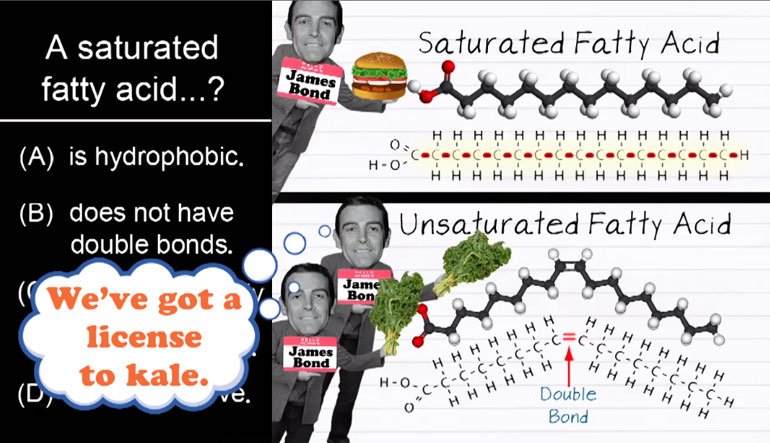
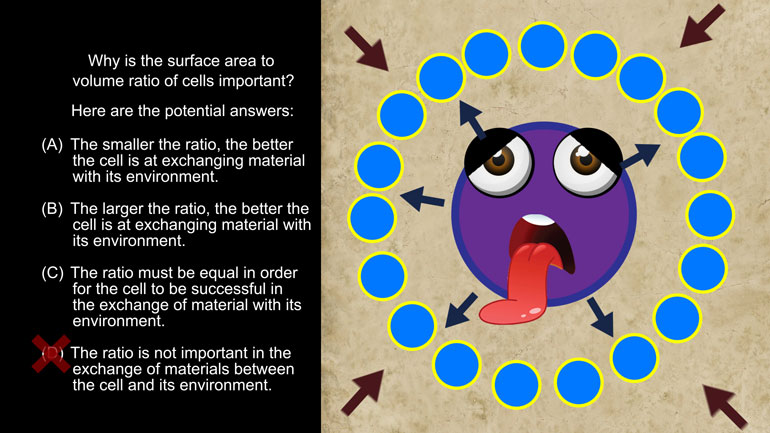
Anything that has a cell (bacteria, listen up!) has phospholipids that keep the cell contained and give it form and shape. Phospholipids protect us...
Chemistry: 4.3 Calculating Subatomic Particles 77 Views
Share It!
Description:
Don't let any atoms near us. We heard that they're "part tickles" and we're having none of that. No sir. You have fun watching this video about calculating subatomic particles. We'll be over here.
Transcript
- 00:03
Verner von Numberstein, the
- 00:06
world-famous scientist and [Numberstein stood on stage]
- 00:07
mathematician, has begun teaching
- 00:09
Heinrich, his one-year-old son, his ABCs
- 00:12
and one-two-threes. However, because the
Full Transcript
- 00:15
Verner feels that nothing should be easy, [Verner teaching Heinrich in a classroom]
- 00:17
he's making Heinrich learn to count
- 00:19
using subatomic particles. Years from now,
- 00:22
Heinrich will have the option of filing [Heinrich working]
- 00:24
a parental abuse lawsuit, but for now, he's
- 00:27
a bit stuck. Well, first Verner introduces
- 00:30
Heinrich to the atomic number. That's the
- 00:32
number that appears right next to every [Atomic number highlighted]
- 00:34
element of the periodic table of
- 00:35
elements. This number, Verner explains,
- 00:38
represents the number of protons in a
- 00:40
given element. A helium atom, for example,
- 00:43
has two protons inside its nucleus, so it [Helium atom with 2 protons inside]
- 00:45
has an atomic number of two. Magnesium
- 00:48
has 12 protons, so it has an atomic
- 00:51
number of 12. Foolium has 143 protons...
- 00:55
Okay, there's no such elements to foolium;
- 00:57
Verner's just trying to keep Heinrich on [Heinrich stands up and falls down]
- 00:59
the toes, which is tough, since the kid
- 01:01
isn't even walking yet. Verner then
- 01:03
points out the mass number. Well, the mass
- 01:05
number is the number of protons (i.e. the
- 01:07
atomic number plus the number of
- 01:10
neutrons), so in a helium atom that has
- 01:12
two neutrons, the mass number is four
- 01:15
(two protons plus two neutrons). In a magnesium [Magnesium protons and neutrons]
- 01:18
atom that has 14 neutrons, the mass
- 01:20
number 26 (12 protons plus 14 neutrons),
- 01:24
and in a foolium atom that has 152
- 01:26
neutrons... Ah there we go, Heinrich's catching
- 01:29
on. Foolium once, shame on you... However
- 01:31
that goes, you know the rest of it.
- 01:34
Verner goes on to tell Heinrich about [Verner discussing isotopes with Heinrich]
- 01:36
isotopes, which are atoms that have the
- 01:38
same atomic number but different mass
- 01:40
numbers. So for example, two boron atoms
- 01:43
would have 10 protons, but if one has 10
- 01:46
neutrons and another has 11, then they're
- 01:48
isotopes, which by the way is the reason
- 01:50
the atomic mass on the periodic table is
- 01:52
always a wonky number--because it's the
- 01:55
average mass of all the different [Definition of atomic mass]
- 01:57
isotopes of that element. Got it? Finally,
- 02:00
Verner gives Heinrich a little pop quiz.
- 02:01
He tells Heinrich that a certain element [Verner giving Heinrich a piece of paper]
- 02:04
has six protons and a mass number
- 02:07
19. He wants to know what this
- 02:09
element is, its atomic number, and how
- 02:12
many neutrons it has. The answer, in case
- 02:14
you're wondering, is that the element is
- 02:17
carbon, because carbon always has six [Carbon highlighted on a periodic table]
- 02:19
protons and since the number of protons
- 02:21
always equals the atomic number, the
- 02:23
atomic number is also six. And since the
- 02:26
mass number is the sum of the protons
- 02:28
and neutrons, then there must be 19-6 (or
- 02:31
thirteen) neutrons. Well, as for for Heinrich's
- 02:34
chances of arriving at the same [Heinrich sitting with a stack of books]
- 02:35
conclusion, well, Verner is quite the optimist.
- 02:37
Although he desperately wants to crank
- 02:39
out the next great child prodigy, Heinrich
- 02:42
has responded to this lesson by sucking
- 02:44
on a big toe, making a few grunting [Heinrich sucking on his toe]
- 02:47
noises and, you know, filling his diaper
- 02:49
for dad, there. For his money, Verner would
- 02:51
rather be inhaling Beryllium. [Verner holding his nose while taking out a diaper]
Related Videos
When you're about to marry the love of your life, not many things could stop you. However, finding out that your future hubby is keeping his crazy...
Here at Shmoop, we work for kids, not just the bottom line. Founded by David Siminoff and his wife Ellen Siminoff, Shmoop was originally conceived...
ACT Math: Elementary Algebra Drill 4, Problem 5. What is the solution to the problem shown?
AP® English Literature and Composition Passage Drill 1, Problem 1. Which literary device is used in lines 31 to 37?
AP® English Literature and Composition Passage Drill 2, Problem 1. What claim does Bacon make that contradicts the maxim "Whatsoever is delig...