ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
ASVAB Videos 95 videos
ASVAB Physical Science 5.2. What happens at higher temperatures?
ASVAB Physical Science 5.3. This is an example of what type of heat transfer?
ASVAB Physical Science 6.1. Low voltage and high resistance will result in which of the following?
ASVAB Physical Science 1.4 184 Views
Share It!
Description:
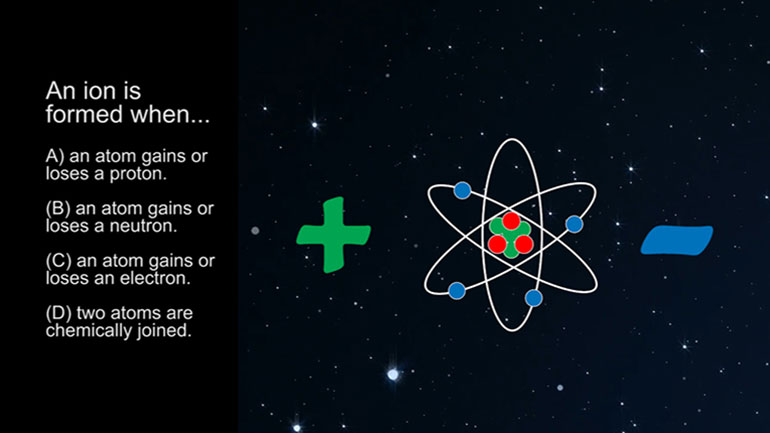
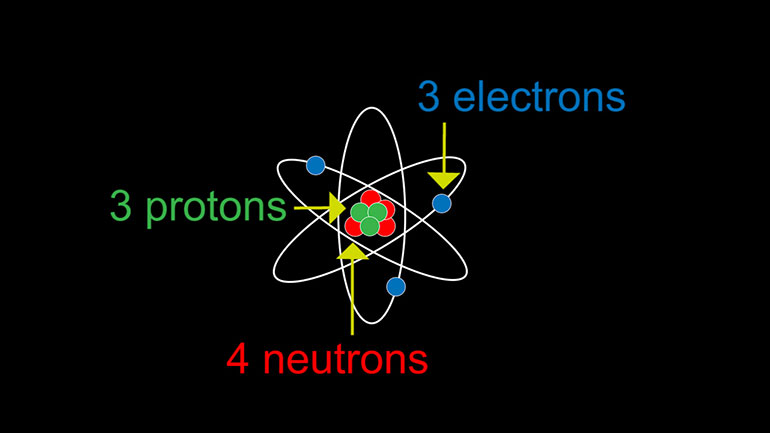
ASVAB Physical Science Drill 1, Problem 4. An atom has three protons, three electrons, and four neutrons. What is the charge of the atom?
Transcript
- 00:00
[ musical flourish ]
- 00:02
And here's your Shmoop du jour, brought to you by atoms.
- 00:05
They make up... everything.
- 00:07
So this is brought to you by, well, everything.
- 00:10
Whoa.
Full Transcript
- 00:11
An atom has three protons, three electrons, and four neutrons.
- 00:15
Three, three, four. What is the charge on the atom?
- 00:18
And here are the potential answers.
- 00:19
[ mumbles ]
- 00:21
Well, figuring out the answer here comes down to basic addition
- 00:23
and subtraction. We just need a quick review.
- 00:26
We've got protons, which carry positive charges.
- 00:29
There are electrons, which carry negative charges.
- 00:32
And there are neutrons, which are particles without a charge.
- 00:36
In our question here, we've got three protons, three electrons, and four neutrons.
- 00:40
Now, it doesn't matter how many neutrons there are in the atom
- 00:42
because neutrons have no charge.
- 00:44
They're the unambitious slackers of the subatomic world.
- 00:47
All we have to do is think of a proton as one
- 00:50
and an electron as a negative one.
- 00:52
So if we take positive three and add it
- 00:55
to negative three, well, we get zero, making option B the correct choice.
- 00:58
And it's important to remember that neutrons don't affect the charge of an atom.
- 01:02
Only the protons and electrons do.
- 01:05
If there are more protons than electrons, well, the atom will have a positive charge.
- 01:09
If there are more electrons than protons, it'll have a negative charge. Duh.
- 01:12
And if there's the same number of each, then we've got
- 01:15
a neutral atom with no charge, like the one in our question and Switzerland.
- 01:19
And if we need to count using our fingers,
- 01:21
we'll go ahead and do it. It's nice; it makes us feel young again.
Related Videos
ASVAB Paragraph Comprehension 1.2 Summary. Which of the following best describes the purpose of this passage?
ASVAB Paragraph Comprehension 1.2 Vocabulary-In-Context. In this passage, the word "illustrious" most nearly means...what?
ASVAB Paragraph Comprehension 1.3 Vocabulary-In-Context. The word "preposterous" most nearly means what?
ASVAB Paragraph Comprehension 2.3 Summary. Which of the following best describes the main idea of this passage?
ASVAB Word Knowledge: Word Roots, Prefixes, and Suffixes Drill 1, Problem 1. Which of these words is closest in meaning to incoherent?