ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Laws of Thermodynamics Videos 14 videos
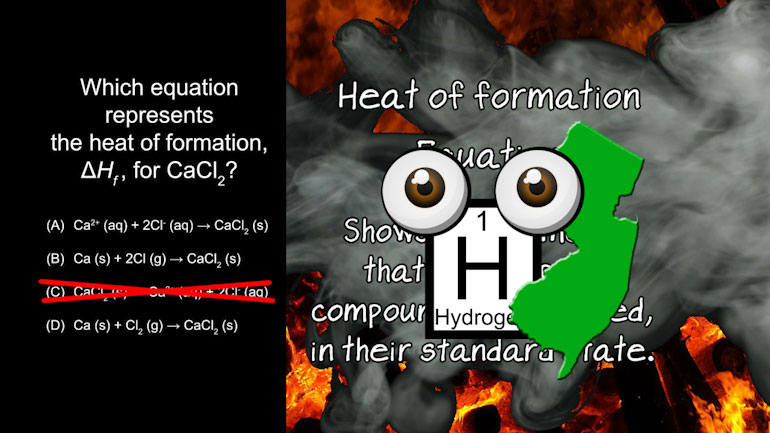
AP® Chemistry: Laws of Thermodynamics Drill 1, Problem 1. Which equation represents the heat of formation for Calcium Chloride?
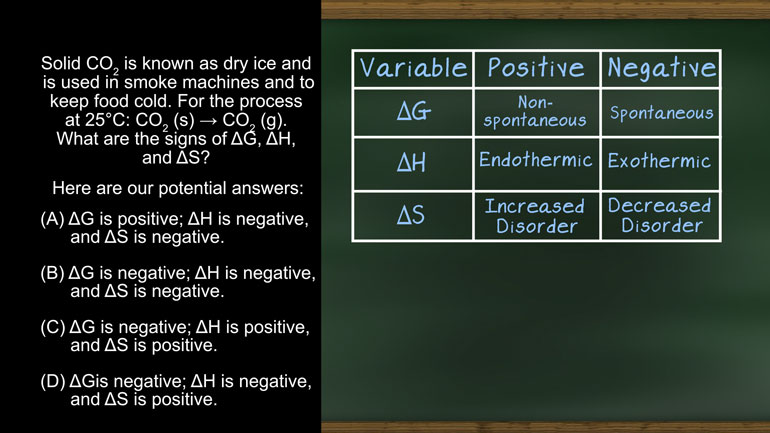
AP Chemistry 2.2 Laws of Thermodynamics. What is the ΔG and the spontaneity of the reacton?
AP Chemistry 3.4 Laws of Thermodynamics 2 Views
Share It!
Description:
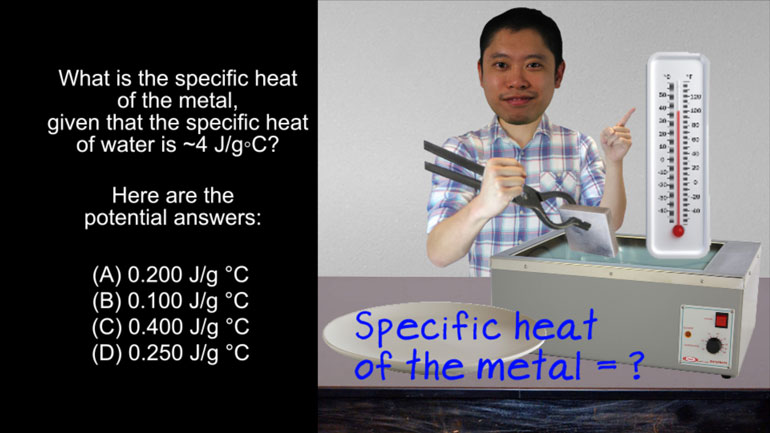
AP Chemistry 3.4 Laws of Thermodynamics. What is the specific heat of the metal?
Transcript
- 00:04
And here's your Shmoop du jour, brought to you by specific heat. [Fire rising]
- 00:07
If it’s too specific, all of those adjectives can get pretty annoying…
- 00:12
Alright, here's the question.
- 00:13
In the lab, Bubba is trying to determine the identity of a metal by its specific heat. [Bubba wearing a lab coat]
- 00:18
Here is the data he collected.
Full Transcript
- 00:24
What is the specific heat of the metal, given that the specific heat of water is ~4 J/g?celcius.
- 00:32
And here are your potential answers.
- 00:35
Hey, maybe if we help Bubba figure out his problem, he’ll give us some free shrimp. [Bubba at the shrimp store]
- 00:41
Nothing motivates like seafood.
- 00:42
All right, let’s take a look at the data that Bubba collected.
- 00:45
It appears that he placed a hotter piece of metal in cooler water, then measured the temperature
- 00:49
of the metal-water mixture. [Bubba with a piece of metal in hot water]
- 00:51
How can we determine the specific heat of the metal with this data?
- 00:54
Well, specific heat, or Cp, refers to the amount of heat required
- 00:58
to raise the temperature of 1 gram of a substance by 1°C.
- 01:02
In order to find the specific heat of the metal, we need to know how much heat was transferred [Piece of metal in a bed of water]
- 01:07
from the metal to the water.
- 01:09
We were given the specific heat of the water, the mass of the water, and the temperature
- 01:12
change of the water, so we can calculate the heat that the water must have absorbed using
- 01:16
the equation heat equals mass times the heat capacity times the change in
- 01:21
temperature.
- 01:22
Due to the law of conservation of energy, we can assume that the same amount of energy
- 01:28
is released by the metal.
- 01:30
Using the same equation, we can calculate our unknown Cp. [Equation for calculating Cp]
- 01:33
All right, now it’s time for our favorite part, doing the math!
- 01:37
Do the math do the math do the maaaath, do the math do the math do the maaaath…
- 01:43
Alright we're done. Choice B is the one we want.
- 01:46
And now, it's time for seafood. [Bubba sat at a table waiting for his order of seafood]
- 01:48
Okay, Bubba, so that’ll be two orders of popcorn shrimp, a shrimp cocktail – anybody
- 01:52
want some garlic bread?
Related Videos
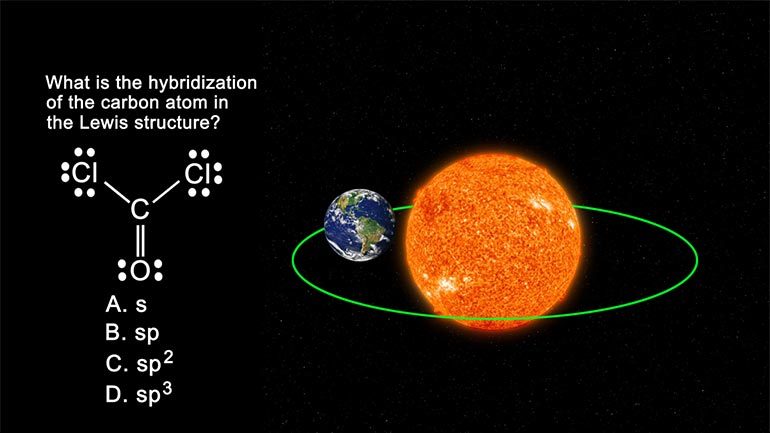
AP Chemistry: Structure of Atoms Drill 1, Problem 5. What is the hybridization of the carbon atom in the Lewis structure?
AP Chemistry DBQ/Free Response. Perform the following calculations.
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?