ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Laws of Thermodynamics Videos 14 videos
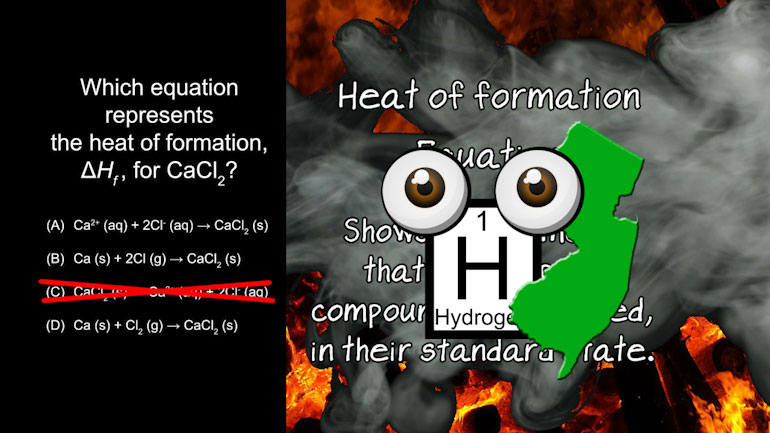
AP® Chemistry: Laws of Thermodynamics Drill 1, Problem 1. Which equation represents the heat of formation for Calcium Chloride?
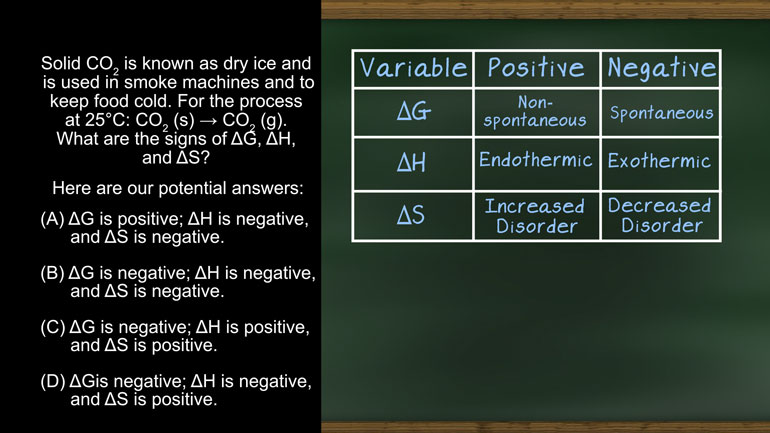
AP Chemistry 2.2 Laws of Thermodynamics. What is the ΔG and the spontaneity of the reacton?
AP Chemistry 1.4 Laws of Thermodynamics 10 Views
Share It!
Description:
AP Chemistry 1.4 Laws of Thermodynamics. Which of the following statements is true?
Transcript
- 00:04
And here’s your Shmoop du jour, brought to you by joules.
- 00:07
They’re the diamond-standard unit of energy, resplendent in their timeless beauty. [Girl wearing jewels and boy cries]
- 00:13
We hear they're sold at Kay.
- 00:14
Okay, here’s our question:
- 00:16
Equal masses of two substances, A and B, each absorb 50 joules of energy.
Full Transcript
- 00:21
If the temperature of A increases by 3 °C, and the temperature of B increases by 6 °C,
- 00:27
which of the following statements is true?
- 00:29
And here are our potential answers: These must be some creepy substances… [Gas appears]
- 00:36
Absorbing all this energy like dementors swallowing your soul… somebody should cast a patronus [Dementor appears]
- 00:42
charm, quick.
- 00:44
What's that?
- 00:45
Any object that increases in temperature is absorbing energy?
- 00:48
Oh….maybe we overreacted a little bit.
- 00:51
So to solve this problem, let’s take a look at this equation. [Finger points to equation]
- 00:53
Here, Q is the amount of heat or energy transferred when a material changes temperature, m is
- 00:59
the material’s mass, Cp is the heat capacity, and delta T is the change in
- 01:05
temperature.
- 01:06
We know from the problem statement that substances A and B have the same mass.
- 01:10
They also both absorb the same amount of energy, 50 joules.
- 01:14
That means the only factors that can differ between them are the change in temperature [Thermometer rising in temperature]
- 01:18
and the heat capacity.
- 01:19
So let’s write that out as an equation, which looks a little something like….this.
- 01:24
And even though it looks complicated, you don't have to be Hermione Granger to solve
- 01:28
this one.
- 01:29
The problem tells us the temperature increase for substance A is 3 degrees Celsius, while [Finger points to temperature increase for substance A]
- 01:33
the temperature increase for substance B is 6 degrees Celsius.
- 01:37
If we reduce this equation and cancel out the units, we can see that the heat capacity
- 01:41
of material A is twice the heat capacity of material B. And that just happens to be answer
- 01:46
choice (A).
- 01:48
Well done. [Girl pours potion]
- 01:49
You know, if you went to Hogwarts, we’re pretty sure you’d be a Ravenclaw, you smarty
- 01:53
pants.
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?