ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Problem Solving and Data Analysis Videos 50 videos
A mole is a unit used in chemistry to measure atoms, molecules, electrons, and other particles in a given amount of substance. We know that the mol...
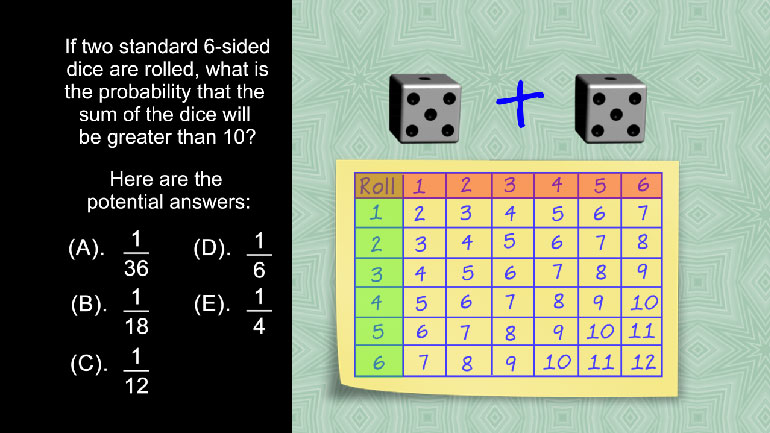
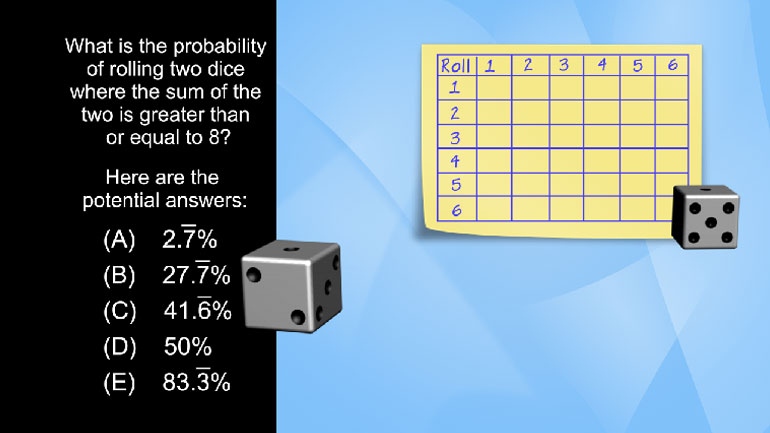
The table above estimates the population increase of a colony of bacteria over the course of five years. Which of the following best characterizes...
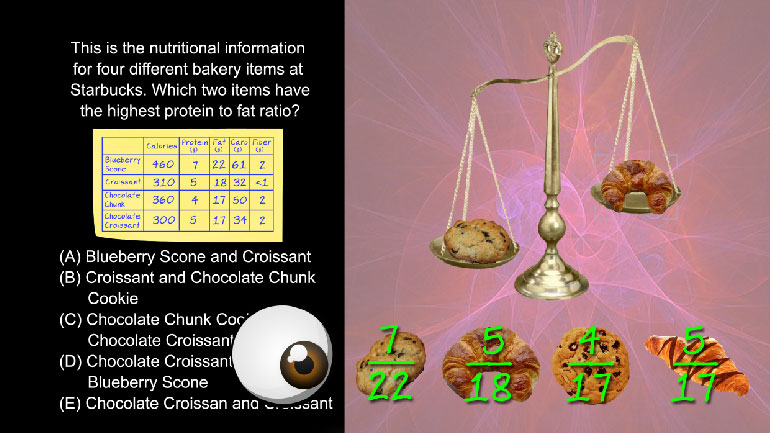
Employees of a candy company surveyed people of different ages and asked them their favorite flavor of candy. One of the surveyors chooses to send...
Using Conversion Factors to Solve for Density in Moles 8 Views
Share It!
Description:
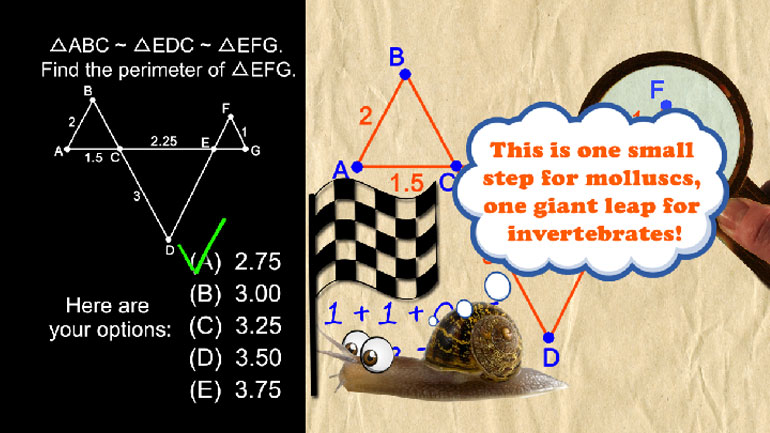
A mole is a unit used in chemistry to measure atoms, molecules, electrons, and other particles in a given amount of substance. We know that the molar mass of mercury is approximately 200 grams per mole. Using a small calibrated test tube containing water, a scientist is able to determine that the volume of a small amount of mercury that he will use in his experiment is 24.5 cubic centimeters. If mercury has a density of 13.5 grams per cubic centimeter, how many moles of mercury are in 24.5 cubic centimeters? Round your answer to one decimal place.
Transcript
- 00:01
Okay s eighty math shmoop er's We've got another siri's
- 00:04
of five questions from you and here we might actually
- 00:07
pull from these formulas This thing this reference information thing
- 00:11
here with the dunce cap on it You know that
- 00:13
thing you used to wear that Okay here we go
Full Transcript
- 00:15
Question One of five Mole different kind of mall is
- 00:21
a unit used in chemistry to measure adam's molecules electrons
- 00:25
and other particles in a given amount of substance We
- 00:28
know that the mueller mass of mercury is approximately two
- 00:30
hundred grams per mall Using a small calibrated test tube
- 00:34
containing water a scientist is able to determine that the
- 00:36
volume of a small amount of mercury that he will
- 00:38
use in his experiment is twenty four point five cubic
- 00:41
centimeters If mercury has a density of thirteen point five
- 00:45
grams per cubic centimeter How many moles and mercury are
- 00:48
in the twenty four point five cubic centimeters there around
- 00:51
your answer Okay Well so how do we think about
- 00:56
this Well let's start with a little prayer Mercury is
- 00:59
a roman god yes A planet and element And now
- 01:03
the subject of a math problem First right What's given
- 01:07
That is specific about the situation right Which is the
- 01:09
volume of twenty four point five cubic centimeters Hey you've
- 01:12
got one free point there A partial credit just for
- 01:15
doing that Then look for the ratio that relates cubic
- 01:18
centimeters to a different unit huh Where would that be
- 01:22
Well in this case the ratio is density and it
- 01:24
relates Cubic centimeters Two grams You just multiplying together making
- 01:29
sure that everything lines up like this And this is
- 01:32
the hard part in this question If you get this
- 01:33
equation right well life will go swimmingly We've got better
- 01:37
Units of cubic centimeters are in the numerator and denominator
- 01:41
So what happens Yes they cancel each other out Conveniently
- 01:45
like a smart people in congress were left with quite
- 01:48
a few grams of mercury here So we should find
- 01:50
something else in our toolbox of information that relates grams
- 01:54
to another unit question Wants units of moles So there's
- 01:58
good direction and go here We've got three hundred thirteen
- 02:00
point seven five grand times One mole over two hundred
- 02:03
grams About one point Seven moles The number you get
- 02:06
in your calculator will be a one point six five
- 02:09
Three seven five of the problem ask you to run
- 02:10
to the nearest tenth just round up to one point
- 02:13
seven and also noticed that we flipped the ratio of
- 02:15
moles program around to convert our final answer In two
- 02:18
moles we were told there were two hundred grams for
- 02:21
everyone mole but we're using moles in the numerator so
- 02:24
we can do this whenever we're presented with this conversion
- 02:29
factor thing Yeah you can look at it right here
- 02:31
it's all free on him up At least this party's
- 02:34
All right we're done answered one point Seven and i
- 02:36
were just going to move on from this weird looking 00:02:38.728 --> [endTime] mole Thanks dude
Related Videos
The table above estimates the population increase of a colony of bacteria over the course of five years. Which of the following best characterizes...
Employees of a candy company surveyed people of different ages and asked them their favorite flavor of candy. One of the surveyors chooses to send...
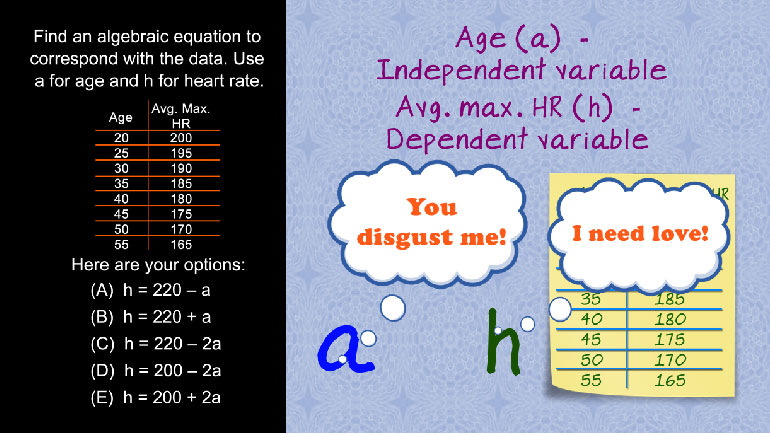
SAT Math 1.1 Algebra and Functions. Find an algebraic equation to correspond with the data.
SAT Math 1.1 Geometry and Measurement. What is the circumference of the circle?
SAT Math 1.1 Numbers and Operations. How many combinations of beverage and cereal can be made?