ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Chemistry Videos 45 videos
AP Chemistry 3.5 Chemical Reaction Rates. How much 85Kr will we need to collect?
Want to pull an Oliver Twist and ask us for more? We've gotcha covered. Head over to /video/subjects/test-prep/ap/ap-chemistry/ for more AP Ch...
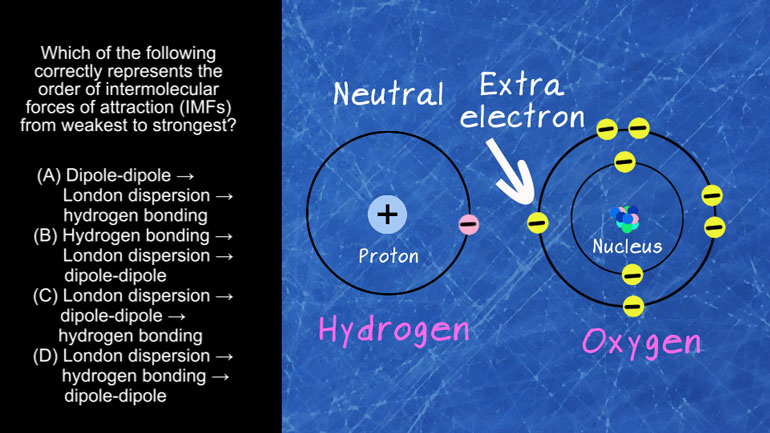
AP Chemistry 1.3 Forming and Breaking Bonds. Which of the following ions are spectator ions?
AP Chemistry 3.5 Rearrangements and Reorganization of Atoms 11 Views
Share It!
Description:
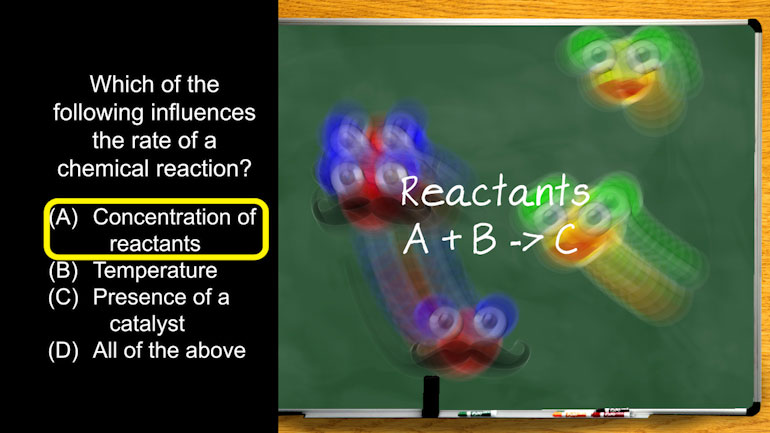
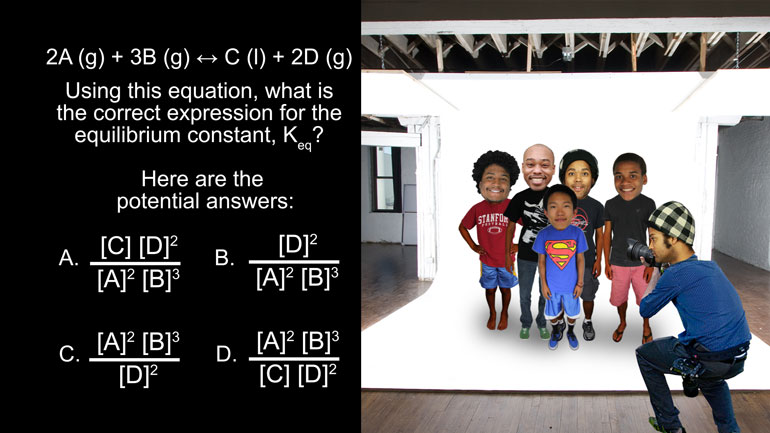
AP Chemistry 3.5 Rearrangements and Reorganization of Atoms. Which of the following is a characteristic of a decomposition reaction?
Transcript
- 00:00
Thank you We sneak and here's your shmoop du jour
- 00:05
brought to you by decomposition because without decomposition our streets
- 00:08
will be filled with garbage Have to imagine isn't it
- 00:12
all right Which of the following is a characteristic of
- 00:14
a decomposition reaction Right here The potential answers Okay well
Full Transcript
- 00:21
this questions about decomposition so it'd probably help to know
- 00:25
what that word means Decomposition means breaking down or splitting
- 00:29
up heart when food waste decomposes it breaks down it's
- 00:33
like that time you broke your mom's favorite vase You
- 00:35
were just decomposing it into a million little pieces there
- 00:38
right way Always knew that it somehow be a valuable
- 00:40
life lesson Well in chemistry of decomposition reaction is a
- 00:43
type of reaction where a single molecule breaks down to
- 00:45
create two or more new atoms or molecules which means
- 00:48
that a decomposition reaction by definition has only one reacted
- 00:53
and that tells us to choice A should be the
- 00:55
correct answer But let's break down the other answers to
- 00:58
make sure since the decomposition reaction always has two or
- 01:01
more products choice people must be wrong and choices cnd
- 01:04
both imply that there are multiple reactant involved which can't
- 01:07
be right either since the decomposition reaction always has only
- 01:09
one reacted Just as we suspected The answer is a
- 01:13
ralph decomposition doesn't take care of our leftover casserole splitting
- 01:16
apart its molecules We certainly will Hopefully you won't eat 00:01:20.784 --> [endTime] so much that we also split our pants
Related Videos
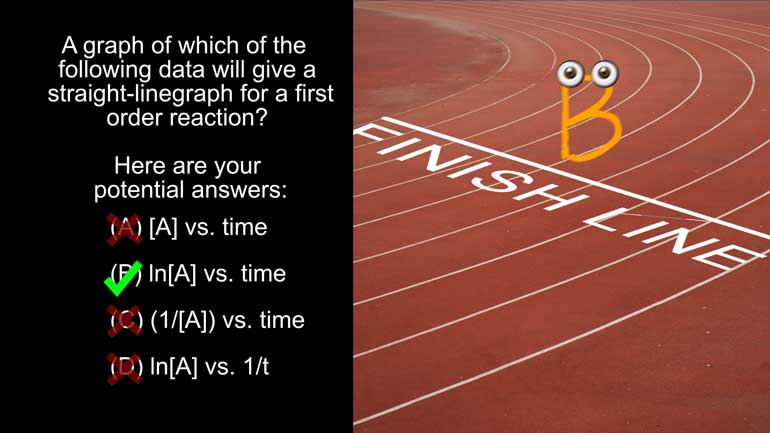
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
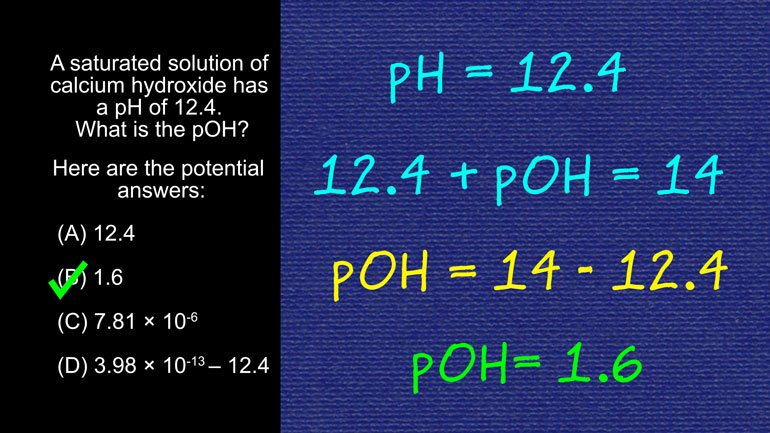
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?