ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Structure and Arrangement of Atoms Videos 9 videos
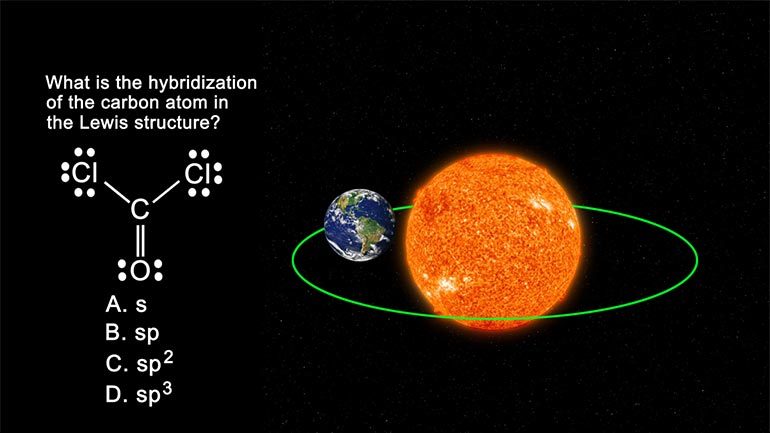
AP Chemistry: Structure of Atoms Drill 1, Problem 5. What is the hybridization of the carbon atom in the Lewis structure?
AP Chemistry 2.5 Structure and Arrangement of Atoms. Under which of the following conditions would a real gas behave more like an ideal gas?
AP Chemistry 3.5 Structure and Arrangement of Atoms. Which of the following is true regarding solids?
AP Chemistry 2.4 Structure and Arrangement of Atoms 35 Views
Share It!
Transcript
- 00:03
Here’s your Shmoop du jour, brought to you by dipoles.
- 00:07
Because we're just dying to pole you in to this video. [Tablet showing a shmoop video]
- 00:10
Here’s the question we have to tackle:
- 00:13
Which of the following molecules has a dipole moment of zero?
- 00:17
And here are our potential answers…
Full Transcript
- 00:23
There are two steps needed to figure out the dipole moment of a molecule. [Woman taking two steps on a piece of furniture]
- 00:26
Step one: figure out which bonds have dipoles, and in what direction.
- 00:30
And step two: cancel out dipoles pointing in opposite directions. [Hands removing dipoles]
- 00:34
Let’s jump right into step one. [Man jumps into swimming pool]
- 00:36
A dipole is just an unequal sharing of electrons in a bond.
- 00:40
Kind of like how our older brother “shares” things with us… [Younger brother trying to grab a teddy off older brother]
- 00:43
Dipoles occur in a bond between an atom with a high electronegativity and an atom with
- 00:48
a lower electronegativity, because atoms with high electronegativity are very selfish. [Atom with high electronegativity]
- 00:55
Just like our older brother, except he's less selfish over electrons, and more over basically everything else
- 01:01
in the world… [Brothers fighting over a toy car]
- 01:03
When we have an electronegative atom in a bond, we draw an arrow along the bond pointing
- 01:08
to the more electronegative atom, which calls them out for hogging the electrons.
- 01:13
Why be subtle, right?
- 01:14
Anyway, this arrow is the dipole of the bond. [Arrow showing dipole of a bond]
- 01:17
And how do we identify electronegative atoms in a bond?
- 01:20
We look at the handy-dandy periodic table, which is essentially Facebook for the elements. [Scientist studying the periodic table on a computer]
- 01:24
Electronegativity follows a regular trend on the periodic table, excluding the Noble
- 01:29
gases, because they can’t be bothered to follow the trends of the common folk.
- 01:34
As we move right or up along the periodic table, electronegativity increases, with Fluorine [Arrows point to Fluorine on a periodic table]
- 01:39
being the most electronegative atom.
- 01:42
And if we're comparing two atoms, the atom further to the right, or further up on the
- 01:46
periodic table is more electronegative.
- 01:50
This means the dipole of a bond between those two atoms will point to the atom further up
- 01:54
or further right on the periodic table.
- 01:56
Okay.
- 01:57
Phew.
- 01:58
So.
- 01:59
…What was the question asking again? Oh right.
- 02:01
Now let’s draw our dipole arrows on our molecules. [Girl drawing arrows on a molecule]
- 02:04
Our molecules without the dipole arrows will look something like this…
- 02:07
Let’s go through them one by one and draw in our dipoles.
- 02:10
First we have H2O. [H2O in chemical form]
- 02:13
The bonds in this molecule are between Hydrogen and Oxygen.
- 02:17
Oxygen is much further right on the periodic table than Hydrogen, so we know that it's
- 02:21
more electronegative.
- 02:22
And if we draw in our bond dipoles....
- 02:26
Any dipoles pointing in opposite directions will cancel. [Dipoles drawn on a H2O molecule]
- 02:30
The dipoles in this molecule point up left and up right.
- 02:33
The left and right cancel, but the up parts add, giving us a leftover dipole.
- 02:37
So answer A is off the table. [H2O answer crossed out]
- 02:40
What about answer B, CO2?
- 02:43
This molecule has bonds between Carbon and Oxygen.
- 02:46
Oxygen is much further right than Carbon on the periodic table so it's more electronegative [Periodic table showing oxygen and carbon]
- 02:50
and our dipoles will point towards oxygen.
- 02:52
One dipole points left and one points right, which means that no one wins this tug of war. [Dipoles pointing in opposite directions]
- 02:57
And if there's no leftover dipole, then yup, this looks like our answer…but let's check [Two boys in a tug of war]
- 03:01
out our other molecules, just for fun.
- 03:04
Because what could be more fun than looking at more dipole moments?
- 03:09
Applying the same logic to the next two molecules, we see that SOCl2 has dipoles pointing towards [SOCl2 molecule shown with dipoles pointing towards oxygen and chlorine]
- 03:15
oxygen and chlorine, like so:
- 03:17
The bond dipoles of this molecule all point in different directions, but nothing points
- 03:22
towards the lone pair.
- 03:24
This means there will be a net arrow pointing opposite that direction… [Finger points to dipole arrow]
- 03:27
And that means this definitely isn't our answer.
- 03:29
Which brings us to our last competitor, D, CHCl3. [Letter D in a boxing ring]
- 03:31
The molecule looks something like this:
- 03:36
Chlorine is much more electronegative than carbon, and hydrogen has a very similar electronegativity
- 03:43
to carbon, so our bond dipoles will look like this: [CHCl3 molecule]
- 03:46
Nice.
- 03:47
Our carbon-hydrogen bond will have no dipole, and our carbon-chlorine bonds will have dipoles
- 03:53
pointing to chlorine.
- 03:54
This gives us a net dipole that looks something like this: [CHCl3 molecule with dipoles pointing to chlorine]
- 03:58
And remember when we started this question approximately twenty years ago, that we were
- 04:02
looking for a molecule with NO dipole.
- 04:05
So this is not the answer.
- 04:06
So that means B is most definitely our answer.
- 04:09
But hey, what better way to spend your time than with molecules? [Scientist using a pipette to transfer blue substance into a flask]
- 04:12
….Point taken.
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?
AP Chemistry 3.1 Laws of Thermodynamics. What is the change in enthalpy of this reaction?