ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Chemistry Videos 54 videos
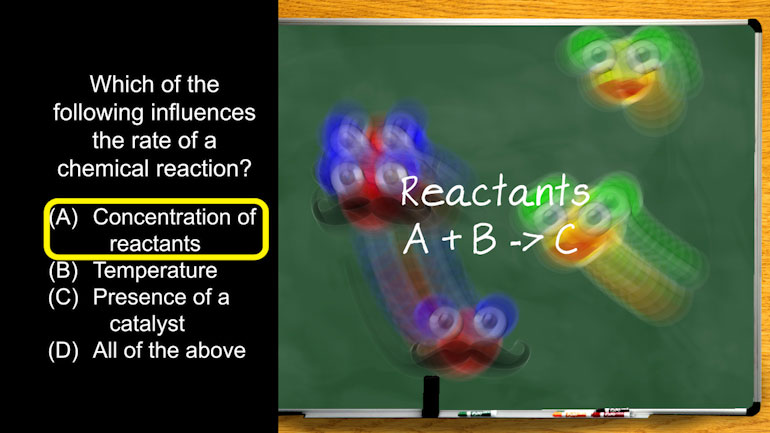
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
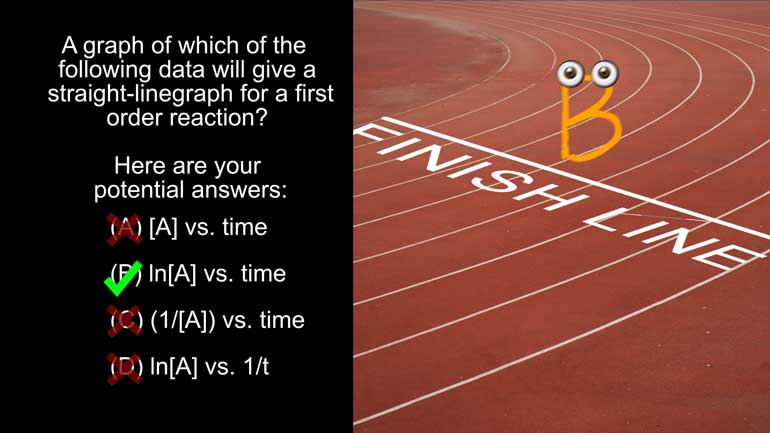
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 1.1 Rearrangement and Reorganization of Atoms 240 Views
Share It!
Description:
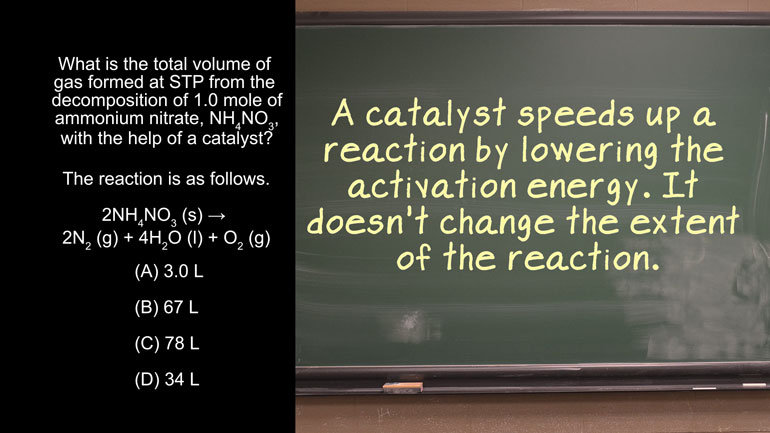
AP® Chemistry: Rearrangement and Reorganization of Atoms Drill 1, Problem 1. Which of the following sets of coefficients correctly balances the below equation in the lowest whole number ratio?
Transcript
- 00:03
Here's your shmoop du jour, brought to you by Hot Tea.
- 00:06
If you spill it in your lap, it will burn for an Oolong time.
- 00:20
Damon decides to make a cup of hot tea by heating water with a gas stove.
- 00:26
The gas stove works through a combustion reaction involving methane gas, or CH4, and oxygen gas.
- 00:35
Which of the following sets of coefficients correctly balances the below equation in the
Full Transcript
- 00:40
lowest whole number ratio?
- 00:43
And here are your potential answers...
- 00:50
So this question is asking us to find the lowest whole number ratio to balance the equation.
- 00:57
If we take a quick look at the answers first, we know C can't be the right answer because
- 01:01
2, 4, 2, and 4 can be reduced to 1, 2, 1, and 2 by dividing all the numbers by 2.
- 01:07
So we can immediately eliminate C.
- 01:13
Now, let's figure out what will make this equation work. Other than, you know... offering
- 01:17
it a possible raise.
- 01:20
Well, in chemistry, according to the law of conservation of mass, there needs to be the
- 01:25
same number of atoms of each element on both sides of the equation.
- 01:29
Which means we can treat the arrow like an equals sign.
- 01:32
For this equation, we know there needs to be the same number of Carbon, Oxygen, and
- 01:36
Hydrogen atoms on the right side of the arrow as there are on the left.
- 01:41
To do this, we can place coefficients in front of the formulas to try to get equal numbers.
- 01:47
Let's try A. If we had 1, 1, 1, and 1...we'd have 1 Carbon on both sides, 4 hydrogens on
- 01:55
the left and 2 on the right...ok, we can stop there. That doesn't work.
- 02:01
B... 1, 2, 1, and 2. 1 Carbon on both sides, 4 hydrogens on the left and 2 times 2 for
- 02:10
4 hydrogens on the right.
- 02:13
2 times 2 is 4 for 4 oxygens on the left...and 2 plus 2 for 4 oxygens on the right.
- 02:21
The coefficients in answer B give us the same number of atoms on the left and right... which
- 02:25
is going to save us a bit of busy work.
- 02:27
Looks like B's our answer.
- 02:29
Now for Damon's next big task: getting the honey for his tea directly from the source...
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
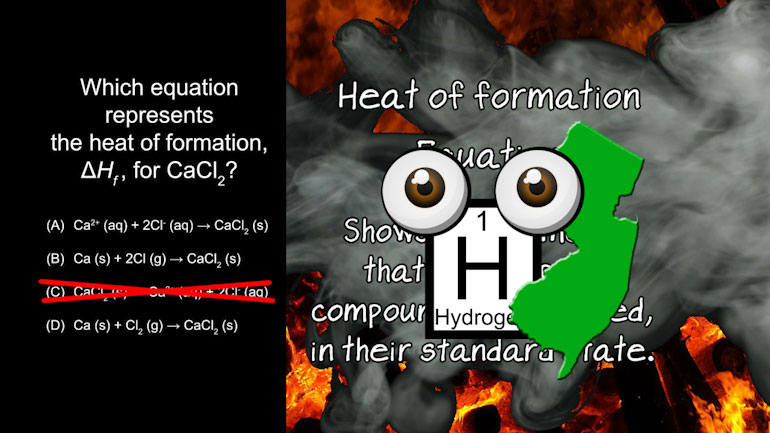
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?