The Theme of Environment in Acids and Bases
Acid Rain
No one likes rain during a parade, but imagine the ensuing chaos if the rain was acidic enough to irritate skin and dissolve dancing clowns.
Instances of acid rain have dramatically increased over the past decades. As you might have heard, this increase corresponds to the increases in industrial and automotive pollution. Your good ol' fashion rain is somewhat acidic (~pH 5.6) because CO2 in the air is able to dissolve into liquid raindrops. The dissolved CO2 reacts with water to form the weak acid bicarbonate. However, pH values for rain as low as 3 have been observed in heavily industrialized parts of the world.
Remember that pH is a logarithmic scale so acid rain has about 1000 times higher concentrations of H+ compared to your run-of-the-mill regular rain. All those reactive protons in the acid raindrops can do some serious damage back here on earth. Lakes and other bodies of freshwater are especially vulnerable. For example, the fish life in thousands of lakes in upper New York and Canada has been nearly wiped out as a result of drastic pH changes to the lake water from acid rain. Changes in soil pH due to acid rain have also killed off massive numbers of trees.
While comparatively less catastrophic, many marble statues of great historical, cultural, and artistic significance have been severely dissolved by routine showers of acid rain.

(Image from here.)
The chemical sources of acid rain have been well characterized and documented (see figure below). The burning of SO2 by industrial plants that use fossil fuels is a major component of acid rain. Gaseous SO2 reacts with water as it dissolves into atmospheric water vapor. This forms sulfurous acid (H2SO3):

In addition, some SO2 oxidizes in the atmosphere forming SO3. This sulfur trioxide dissolves into rain drops forming another strong acid, H2SO4:
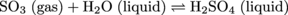
Coal-burning power plants produce particularly high levels of nitrogen oxides that end up in acid rain as nitric acid:


(Image from here.)
There are non-manmade sources of sulfur and nitrogen oxides in the atmosphere that can also dissolve into rain. In particular, volcanoes and lightening bolts emit these chemicals, albeit in tiny quantities compared to the known amounts of sulfur and nitrogen oxides emitted from man-made sources of pollution.
The link between man-made sources of pollution and acid rain is further bolstered by the following observations. First, relatively short exposures to acid rain can kill off entire fish populations that took millennia to grow. Those fish populations would have never been present to begin with if constant, natural sources of sulfur and nitrogen oxides were capable of killing off fish-life. Second, even stronger evidence establishing the connection of man-made pollutants and acid rain comes from the observation that the locations where acid rain has been observed correlate with the locations of man-made, pollution-rich industrial centers. In locations with enduring winds, instances of acid rain are highest precisely in the locations where they would be predicted based on wind patterns. These location correlations are so strong that the probability that acid rain is the result of man-made pollution is ridiculously high.
So what have we done about it? The first step to solving any problem begins with acknowledging that there is a problem and that there are substantive steps that can be taken to solve or mitigate the expected future effects of the problem.
Luckily, several steps have been implemented to reduce sulfur and nitrogen oxides in the atmosphere. One of the first initiatives was to require catalytic converters to be installed on all newly manufactured cars starting around 1975. The catalytic converter converts several nitrogen oxides that are present in gasoline engine exhaust to relatively harmless nitrogen gas (N2) and oxygen gas (O2). Acidic emissions from power plants have also been reduced by the use of emission scrubbers that remove sulfur oxides before the emissions exit the plant's smoke stacks. One type of scrubber consists of lime (CaO) that reacts with the SO2 in the emissions to form calcium sulfite:

Unfortunately, CaSO3 is a fairly useless compound and it must be disposed of in a landfill. On the flip side though, storing SO2 in CaSO3 is a much better alternative to storing it in the atmosphere where it is free to reek acid-style havoc on innocent little rain drops. Hooray for smoke stack scrubbers. While these measures have significantly reduced instances of acid rain in very recent years, challenges remain to curbing emissions further. New technologies will be needed to ensure our future parades stay acid rain free.